Rawalpindi : Having one of the highest prevalence rate of hepatitis-C in the world, Pakistan needs to work religiously both for treatment of patients and for screening of people if we want to eliminate the disease by 2030 as has been pledged by the World Health Assembly of which Pakistan is a signatory.
(more…)
20/03/2017
After a long waiting for, and hard discussions about pricing, we are now pleased to have the first pangenotypic (for all genotypes) approved in Quebec. (more…)
 Here are two new medicines that have just been approved by Health Canada on August 17th and offer more therapeutic options for treating physicians, depending on the specific case of each patient. (more…)
Here are two new medicines that have just been approved by Health Canada on August 17th and offer more therapeutic options for treating physicians, depending on the specific case of each patient. (more…)
 By Melisa Dickie
By Melisa Dickie
As deaths from many communicable diseases continue to decline globally, deaths caused by viral hepatitis have now surpassed all other chronic infectious diseases, including HIV/AIDS, malaria and tuberculosis. (more…)
OBJECTIVES:
To examine the association between coffee, including caffeinated and decaffeinated coffee, with hepatocellular carcinoma (HCC) and assess the influen ce of HCC aetiology and pre-existing liver disease. (more…)
ce of HCC aetiology and pre-existing liver disease. (more…)
Capahc is pleased to annonce good new for people living with hepatitis C.
The province of Quebec is now reimbursing ZEPATIER® (elbasvir/grazoprévir) for hepatitis C patients. (more…)
On January 17, 2017, Health Canada approved the use and sale of a point-of-care antibody test for screening people for exposure to hepatitis C virus (HCV). The test is called the OraQuick HCV rapid antibody test and is manufactured by Orasure Technologies in Bethlehem, Pennsylvania. It will be distributed in Canada by KNS Canada. (more…)
Epclusa, is the first once daily hep C treatment regardless of genotype. (more…)
Calvin Q. Pan, M.D., Zhongping Duan, M.D., Erhei Dai, M.D., Shuqin Zhang, M.D., Guorong Han, M.D., Yuming Wang, M.D., Huaihong Zhang, M.D., Huaibin Zou, M.D., Baoshen Zhu, M.D., Wenjing Zhao, M.D., and Hongxiu Jiang, M.D., forthe China Study Group for the Mother-to-Child Transmission of Hepatitis B* (more…)
CBC News Posted: Jun 20, 2016 2:50 PM ET Last Updated: Jun 20, 2016 4:19 PM ET
‘Victims are being re-victimized,‘ says man whose wife died before receiving any compensation (more…)
194 Member States commit to eliminating viral hepatitis at World Health Assembly
LONDON, May 30, 2016 /CNW/ – On 28 May, 194 Member States made a historic commitment to eliminate viral hepatitis by 2030. At the 69th World Health Assembly, governments unanimously voted to adopt the first ever Global Viral Hepatitis Strategy, signalling the greatest global commitment in viral hepatitis to date. (more…)
 Background and Aims: Preliminary reports indicate that in Liver Transplant candidates listed for Hepatitis C virus (HCV) decompensated cirrhosis, Direct-Acting Antivirals (DAA) therapy may result in liver function improvement. Whether this clinical improvement translates into the delisting of some patients is presently unknown. Therefore, the major object of this study was to assess if and which patients can be first inactivated due to clinically improvement and subsequently delisted in a real life setting. (more…)
Background and Aims: Preliminary reports indicate that in Liver Transplant candidates listed for Hepatitis C virus (HCV) decompensated cirrhosis, Direct-Acting Antivirals (DAA) therapy may result in liver function improvement. Whether this clinical improvement translates into the delisting of some patients is presently unknown. Therefore, the major object of this study was to assess if and which patients can be first inactivated due to clinically improvement and subsequently delisted in a real life setting. (more…)
Good news for hepatitis C patients in Quebec:
• All patients with chronic hepatitis C, genotype 1 and fibrosis stage F2-F4 will have access to Harvoni and Holkira Pak from March 24, 2016. (more…)
 A Gilead pangenotypic treatment (1,2,4,5,6) is coming soon, maybe the end of the genotyping…
A Gilead pangenotypic treatment (1,2,4,5,6) is coming soon, maybe the end of the genotyping…
A simple treatment regimen that is effective in a broad range of patients who are chronically infected with the hepatitis C virus (HCV) remains an unmet medical need. (more…)
 Hepatitis C virus is present in large enough quantities in the rectal fluid of men with HIV and hepatitis C co-infection to permit HCV transmission without the presence of blood, researchers from the Icahn School of Medicine, Mount Sinai Hospital, New York, reported on Sunday at the 2015 AASLD Liver Meeting in San Francisco. (more…)
Hepatitis C virus is present in large enough quantities in the rectal fluid of men with HIV and hepatitis C co-infection to permit HCV transmission without the presence of blood, researchers from the Icahn School of Medicine, Mount Sinai Hospital, New York, reported on Sunday at the 2015 AASLD Liver Meeting in San Francisco. (more…)
- TECHNIVIE provides an opportunity to treat adults who have genotype 4 (GT4) chronic hepatitis C virus (HCV) infection without cirrhosis, a population historically considered difficult–to-treat
- TECHNIVIE is the first and only all-oral, interferon-free, direct-acting antiviral treatment approved in Canada for adult patients with GT4 chronic HCV infection
- Approval is supported by a Phase II clinical trial of 135 chronic HCV GT4 patients, which demonstrated 100 percent sustained virologic response rates at 12 weeks post-treatment (SVR12) in patients who took TECHNIVIE with ribavirin (RBV)
(more…)
 Originally published in the New England Journey of Medecine
Originally published in the New England Journey of Medecine
Salim S. Abdool Karim, M.B., Ch.B., Ph.D., Quarraisha Abdool Karim, Ph.D., Ayesha B.M. Kharsany, Ph.D., Cheryl Baxter, Ph.D., Anneke C. Grobler, Ph.D., Lise Werner, M.Sc., Angela Kashuba, Pharm.D., Leila E. Mansoor, Ph.D., Natasha Samsunder, B.Tech., Adrian Mindel, M.D., and Tanuja N. Gengiah, Ph.D. for the CAPRISA 004 Trial Group (more…)
 This document could be for you, because the Joint Committee wants to hear from you, about a financial surplus. (more…)
This document could be for you, because the Joint Committee wants to hear from you, about a financial surplus. (more…)
 This all-oral therapy without interferon and ribavirin was approved today by Health Canada. (more…)
This all-oral therapy without interferon and ribavirin was approved today by Health Canada. (more…)
 The CAPAHC is pleased with the Québec Ministry of Health and Social Services’ decision to add Harvoni and Holkira Pak to the list of medications that are covered by the RAMQ. (more…)
The CAPAHC is pleased with the Québec Ministry of Health and Social Services’ decision to add Harvoni and Holkira Pak to the list of medications that are covered by the RAMQ. (more…)

This year, focus on prevention.
To read more
 Clinical criterion:
Clinical criterion:
Liver fibrosis stage of ≥ 2. (more…)
Treatment with interferon (IFN) and ribavirin (RBV) significantly impairs quality of life and other patient-reported outcomes (PROs). Patient experience with IFN- and RBV-free anti-HCV (hepatitis C virus) regimens has not been reported. We assessed PROs in patients treated with ledipasvir and sofosbuvir (LDV/SOF) with and without RBV.
(more…) — Harvoni Provides High Cure Rates (SVR12), Shortens Treatment Duration and Eliminates Need for Interferon and Ribavirin — (more…)
— Harvoni Provides High Cure Rates (SVR12), Shortens Treatment Duration and Eliminates Need for Interferon and Ribavirin — (more…)
 New all-oral, short-course (12 weeks), interferon-free treatment available in Canada for genotype 1 hepatitis C patients
New all-oral, short-course (12 weeks), interferon-free treatment available in Canada for genotype 1 hepatitis C patients
– In Phase 3 clinical trials, HOLKIRA PAK (with or without ribavirin) cured an overall 97 percent of GT1 HCV patients; additionally, 98 percent of patients completed treatment
– HOLKIRA PAK was evaluated in more than 2,300 patients in over 25 countries, demonstrating consistently high cure rates across a large and diverse patient population
(more…)
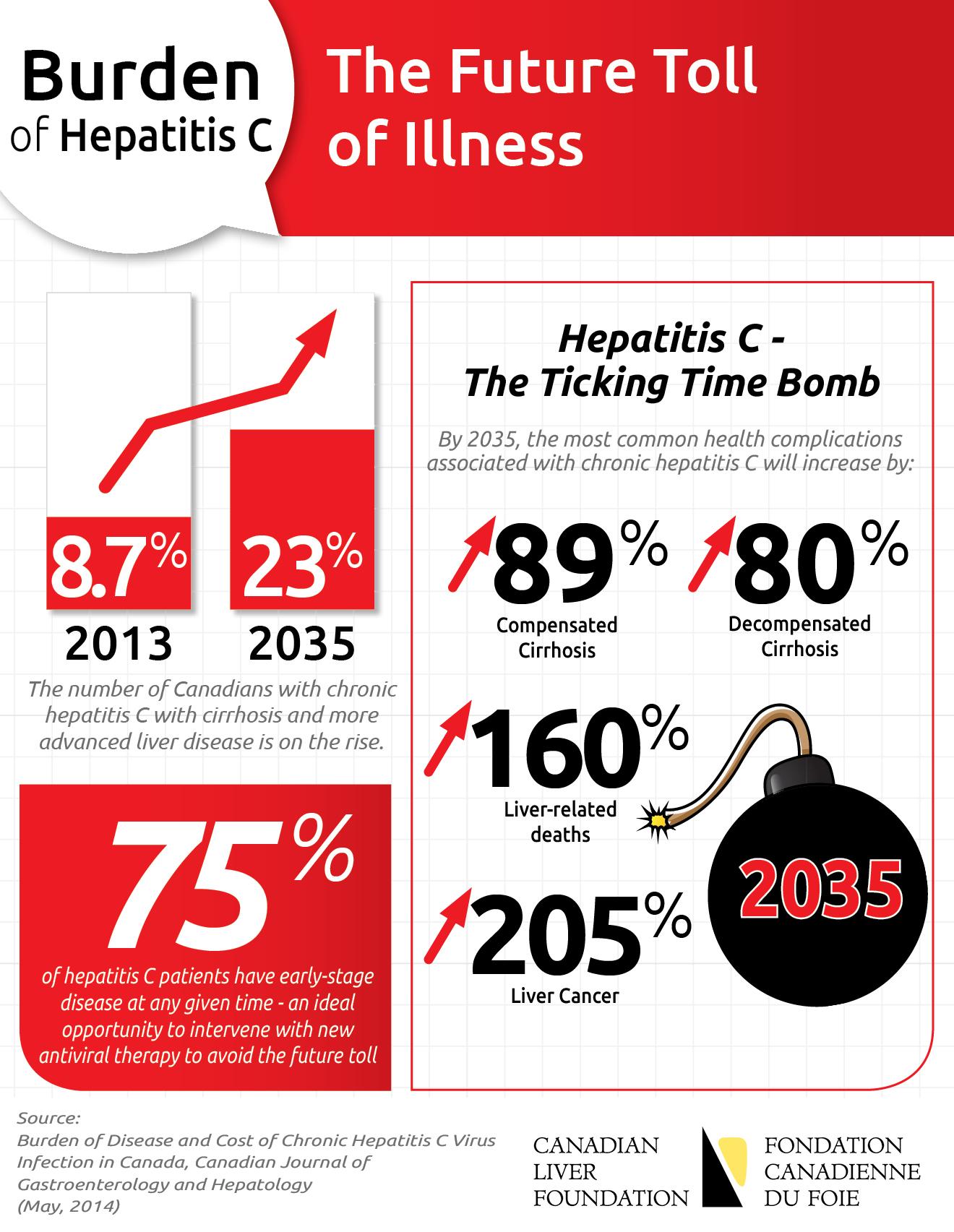
 Montreal (Quebec), March 31, 2014 – New hepatitis C (HCV) treatment regimens have advanced rapidly in recent years, including the recent Health Canada approval of interferon-free therapy. To support patients to access these newest options, Pendopharm, a division of Pharmascience Inc., today announced that it has received a Priority Review designation from Health Canada for the first stand-alone ribavirin tablet for the Canadian market. (more…)
Montreal (Quebec), March 31, 2014 – New hepatitis C (HCV) treatment regimens have advanced rapidly in recent years, including the recent Health Canada approval of interferon-free therapy. To support patients to access these newest options, Pendopharm, a division of Pharmascience Inc., today announced that it has received a Priority Review designation from Health Canada for the first stand-alone ribavirin tablet for the Canadian market. (more…)
 TORONTO July 23 2014 /CNW/ – As we mark World Hepatitis Day (July 28th) people living with hepatitis C virus (HCV) have reason to feel hopeful. New cures for HCV are now available and more effective treatment options will hit the market in the future. Despite the increased hope there is concern. New cures are very expensive raising questions about patient access to treatment. (more…)
TORONTO July 23 2014 /CNW/ – As we mark World Hepatitis Day (July 28th) people living with hepatitis C virus (HCV) have reason to feel hopeful. New cures for HCV are now available and more effective treatment options will hit the market in the future. Despite the increased hope there is concern. New cures are very expensive raising questions about patient access to treatment. (more…)
 A Phase 2 clinical trial is now underway to evaluate sofosbuvir (Sovaldi) plus ribavirin for children and adolescents, ages 3-17 years, with hepatitis C virus genotypes 2 or 3. Another study will follow to test the forthcoming sofosbuvir/ledipasir coformulation in pediatric patients. (more…)
A Phase 2 clinical trial is now underway to evaluate sofosbuvir (Sovaldi) plus ribavirin for children and adolescents, ages 3-17 years, with hepatitis C virus genotypes 2 or 3. Another study will follow to test the forthcoming sofosbuvir/ledipasir coformulation in pediatric patients. (more…)
 Here are two new medicines that have just been approved by Health Canada on August 17th and offer more therapeutic options for treating physicians, depending on the specific case of each patient.
Here are two new medicines that have just been approved by Health Canada on August 17th and offer more therapeutic options for treating physicians, depending on the specific case of each patient. 
 ce of HCC aetiology and pre-existing liver disease.
ce of HCC aetiology and pre-existing liver disease.  Background and Aims: Preliminary reports indicate that in Liver Transplant candidates listed for Hepatitis C virus (HCV) decompensated cirrhosis, Direct-Acting Antivirals (DAA) therapy may result in liver function improvement. Whether this clinical improvement translates into the delisting of some patients is presently unknown. Therefore, the major object of this study was to assess if and which patients can be first inactivated due to clinically improvement and subsequently delisted in a real life setting.
Background and Aims: Preliminary reports indicate that in Liver Transplant candidates listed for Hepatitis C virus (HCV) decompensated cirrhosis, Direct-Acting Antivirals (DAA) therapy may result in liver function improvement. Whether this clinical improvement translates into the delisting of some patients is presently unknown. Therefore, the major object of this study was to assess if and which patients can be first inactivated due to clinically improvement and subsequently delisted in a real life setting.  A Gilead pangenotypic treatment (1,2,4,5,6) is coming soon, maybe the end of the genotyping…
A Gilead pangenotypic treatment (1,2,4,5,6) is coming soon, maybe the end of the genotyping… Hepatitis C virus is present in large enough quantities in the rectal fluid of men with HIV and hepatitis C co-infection to permit HCV transmission without the presence of blood, researchers from the Icahn School of Medicine, Mount Sinai Hospital, New York, reported on Sunday at the
Hepatitis C virus is present in large enough quantities in the rectal fluid of men with HIV and hepatitis C co-infection to permit HCV transmission without the presence of blood, researchers from the Icahn School of Medicine, Mount Sinai Hospital, New York, reported on Sunday at the  Originally published in the New England Journey of Medecine
Originally published in the New England Journey of Medecine This document could be for you, because the
This document could be for you, because the  This all-oral therapy without interferon and ribavirin was approved today by Health Canada.
This all-oral therapy without interferon and ribavirin was approved today by Health Canada.  The CAPAHC is pleased with the Québec Ministry of Health and Social Services’ decision to add
The CAPAHC is pleased with the Québec Ministry of Health and Social Services’ decision to add 
 Clinical criterion:
Clinical criterion: 
 — Harvoni Provides High Cure Rates (SVR12), Shortens Treatment Duration and Eliminates Need for Interferon and Ribavirin —
— Harvoni Provides High Cure Rates (SVR12), Shortens Treatment Duration and Eliminates Need for Interferon and Ribavirin — 
 Montreal (Quebec), March 31, 2014 – New hepatitis C (HCV) treatment regimens have advanced rapidly in recent years, including the recent Health Canada approval of interferon-free therapy. To support patients to access these newest options, Pendopharm, a division of Pharmascience Inc., today announced that it has received a Priority Review designation from Health Canada for the first stand-alone ribavirin tablet for the Canadian market.
Montreal (Quebec), March 31, 2014 – New hepatitis C (HCV) treatment regimens have advanced rapidly in recent years, including the recent Health Canada approval of interferon-free therapy. To support patients to access these newest options, Pendopharm, a division of Pharmascience Inc., today announced that it has received a Priority Review designation from Health Canada for the first stand-alone ribavirin tablet for the Canadian market. 
 A Phase 2 clinical trial is now underway to evaluate sofosbuvir (Sovaldi) plus ribavirin for children and adolescents, ages 3-17 years, with hepatitis C virus genotypes 2 or 3. Another study will follow to test the forthcoming sofosbuvir/ledipasir coformulation in pediatric patients.
A Phase 2 clinical trial is now underway to evaluate sofosbuvir (Sovaldi) plus ribavirin for children and adolescents, ages 3-17 years, with hepatitis C virus genotypes 2 or 3. Another study will follow to test the forthcoming sofosbuvir/ledipasir coformulation in pediatric patients.